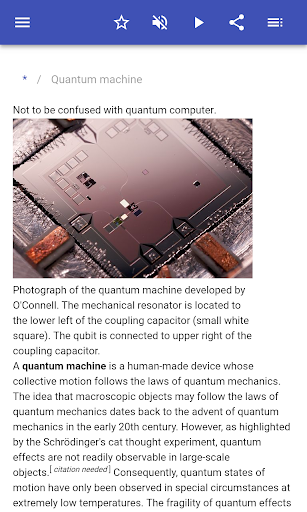
Description
For example, a photon is a single quantum of light (or of any other form of electromagnetic radiation), and can be referred to as a "light quantum". Similarly, the energy of an electron bound within an atom is also quantized, and thus can only exist in certain discrete values. Atoms and matter in general are stable because electrons can only exist at discrete energy levels in an atom. Quantization is one of the foundations of the much broader physics of quantum mechanics. Quantization of the energy and its influence on how energy and matter interact (quantum electrodynamics) is part of the fundamental framework for understanding and describing nature.
User Reviews for Quantum Physics 1
-
for Quantum Physics
Quantum Physics app provides a clear explanation of quantization. Great for understanding discrete values in physics. Highly recommended for students.